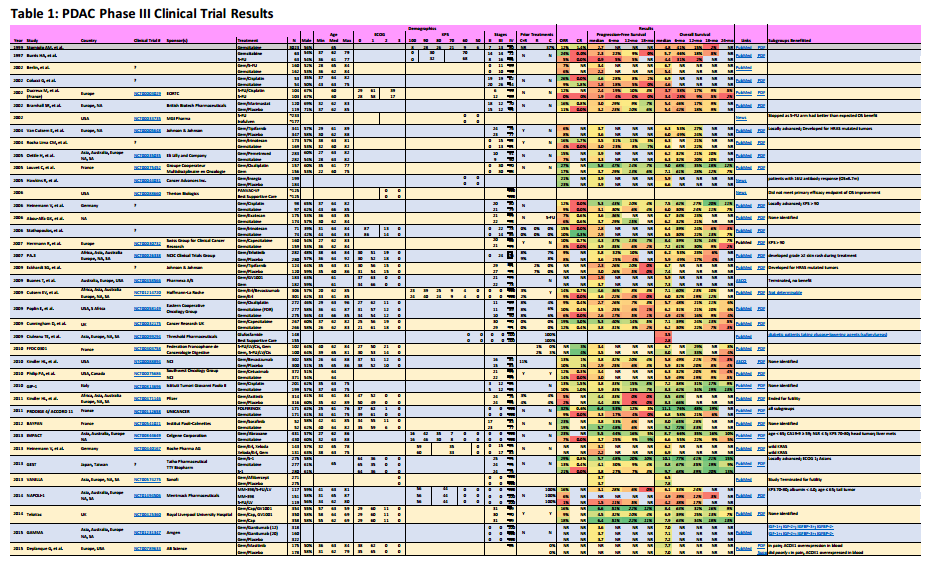
Overview
Metastatic pancreatic adenocarcinoma patients (PDAC) don’t have a lot of good options. In this post, I’ve summarize the completed chemotherapy-based phase III trials for metastatic patients, providing links to publications, outlining the survival statistics, and identifying key subgroups that may have benefited from the treatment.
From a patient’s perspective, not much progress has been made on new treatments. It’s not for a lack of trying. Over 90% of phase 3 clinical trials result in no added benefit.
However, I believe that these treatments are effective on a minority of patients. The data used to measure effectiveness (like median OS) ignores outstanding responses to less than half the patients. I propose that looking at longer term survival rates gives clues to treatments effective on a minority of patients. Further digging into the results by researchers can uncover reasons behind the effectiveness and help us match treatments to patients.
Key Points for Patients
- Several treatments available. We need to better match the right treatment to the right patients. Some studies have published information about subgroups that benefited substantially from treatment in a trial. This can help you choose whether that treatment is right – or wrong – for you.
- A patient’s treatment goals should determine which trial results are important.
- Directly comparing results between trials is misleading due to the fact that patient selection is key to the results and each trial has its own selection criteria.
- Results in italics are values I’ve read from published graphs, not specifically published by the study paper.
- Results in bold are for treatments often used for pancreatic cancer.
Observations
In reviewing the information to put together this information, I’ve formed some interesting opinions that I’ll share.
First, I should state that the results provided here are for the actual patients that were under clinical trial. Your results will almost certainly not be “typical”. There are uncertainties in the outcomes that are hopefully minimized because these are phase 3 clinical trials with large numbers of patients. One of my goals here is to identify the best performing treatments that can maximize your outcomes. If we can identify some treatments that work best for patients in your particular situation, this posting has done its job.
This summary lists very little about side effects of the treatments. For some, this can also be a big factor in a treatment decision. If you are in poorer health, try looking for the best performing trials that included patients with poor health (ECOG 2/3 or KPS≤70).
In the data listed, numbers provided directly from the clinical trial results are listed in normal fonts. Rates in Italics have been interpolated from graphs provided by the clinical trials publications.
Click on the following picture to show a PDF file with the results.
Subgroups Benefited
I think this is the most useful information available from the data I’ve collected.
Some clinical trial reports are superior to others. Take for instance, the reports for FOLFIRINOX and Gemcitabine/Abraxane. Both of these reports include what are called forest plots to identify subgroups of patients that did well on one treatment versus the other.
The FOLFIRINOX forest plot is here (I provide the link because I’m unsure whether I can legally embed the figure, which might be clearer). For each subgroup listed, a hazard ratio has been computed (square box), with 95% confidence intervals shown (horizontal line). Subgroups listed that have the entire confidence interval (horizontal line) to the left of 1.0 (which is just about everything) performed better under FOLFIRINOX than Gemcitabine. This kind of information can be vital to patients who belong to one of these subgroups when they’re trying to decide on a particular treatment.
In the column Subgroups Benefited, I try to list groups indicated within these reports that did benefit from one treatment arm. Sometimes, a follow-on report has the information and I have provided hyperlink in the column for these cases.
ORR
This is the percentage of patients that had at least a 20% reduction in the size of their tumor. FOLFIRINOX has the best ORR at 32%. Understand that 2/3d’s of treated patients did not have their tumors shrink using the best treatment available.
The key is finding out why these patients had their tumors shrink. Are you more likely to belong in this group? Again, this is why the subgroup analysis is important.
CR
If your treatment goal is to remove all metastases to enable a chance at surgery, CR (Complete Response) is your result of interest. These are pitifully small numbers. But what this tells me is that some small subgroup of patients do respond remarkably well to some treatments. One example might be BRCA2 mutation patients that respond extraordinarily well to platinum-based treatments [reference needed].
PFS
If your treatment goal something like living as long as possible without worsening symptoms., this might be your series of results to look at. Often, only the median is reported, but I favor looking at the 12- or 18-month PFS percentages. This tells me how many patients had a sustained response to the treatment. Their tumor shrunk and they’re tolerating the treatment.
OS
This is usually a clinical trial’s primary end statistic. Median numbers are what researchers compare but I find them largely irrelevant to patients. I want to see the 18- and 24-month OS percentages. These are the long-term survivors. Compare the PFS and OS percentages and know what fraction of survivors are still benefiting from the treatment.
Clinical Trial Progress
Often we patients are frustrated by the lack of progress towards more effective treatments. Here I’ve documented over 38 different chemotherapy phase III clinical trials since 2002 (I’m certain there are more). Only four of these trials led to approval, a success rate of about 10%.
In general, these are drugs that had successful phase II results and then failed during phase III. First, it helps to demonstrate that there is still significant effort being put into new treatments. But second, how many very promising treatments fail to show patient benefit.
Interesting Results
When a trial fails to show benefit, often that is the end of the road. However, sometimes the researchers see interesting things buried in their data. Here are a few I’ve uncovered.
Gemcitabine/S-1
Here’s a stellar performer that I think should be approved pronto! For Asians. And therein lies one of our problems with the clinical trial system. It is stacked in favor of Caucasians. This combination works well and is well tolerated by Asians only. Caucasians – not so much.
Gemcitabine/Cisplatin & Gemcitabine/Oxaliplatin
The trial results for these platinum-based chemotherapies did not identify any subgroups that performed well, but it has since been recognized that many patients with a wild-type BRCA2 mutation (about 5-10% of all pancreatic cancers) have responded quite well (2009 Dramatic Response; 2011 Complete Response; 2012 Complete Response; 2014 Meta-analysis). I credit my complete response to Gemcitabine/Cisplatin because of my BRCA2 mutation.
Gemcitabine/Masitinib
According to the traditional measurement criteria (median PFS and/or OS), this trial failed miserably. A closer reading of the results (kudos researchers!) identifies that PDAC patients with overexpression of ACOX1 in their blood did much better than others (Figure). What’s less clear is whether this finding also says that patients with an overexpression of ACOX1 in their blood should avoid Gemcitabine monotherapy.
Gemcitabine/Ganitumab
Another research report (behind a paywall, so not fully reviewed by me) of a failed clinical trial. But a later closer analysis of the results in another paper delivers news of biomarkers (IGF-1+; IGF-2+; IGFBP-3+; IGFBP-2-) that identified a subgroup of patients that survived 3X longer than the others.
Gemcitabine/Erlotinib
Erlotinib (aka Tarceva) was expected to target tumors that overexpress EGFR, like pancreatic cancer. While the EGFR status of patients did not seem to affect the outcomes, development of rashes did. The more severe the rash, the better the outcomes (Figure). In fact, some oncologists are increasing the doses of erlotinib until a rash develops.
Summary
There have been a surprising number of phase III clinical trials for metastatic pancreatic adenocarcinoma. Most do not pan out. And researcher responsibilities should not stop there. It appears unlikely we will soon find a treatment that works for most pancreatic cancer tumors. More effort is being made to identify subgroups that respond well, but that information is not widely disseminated to patients. Hopefully this blog entry will bridge some of that gap.

David, this is fascinating. Could you email all this to me? I cannot print from m fb. Thanks, marci
You can email me at david at pancanology dot com with your contact information.